number of electrons in bromine|Iba pa : Pilipinas The atomic number of each element increases by one, reading from left to . Find & Download Free Graphic Resources for Business Cartoon. 2,187,000+ Vectors, Stock Photos & PSD files. Free for commercial use High Quality Images
number of electrons in bromine,Element Bromine (Br), Group 17, Atomic Number 35, p-block, Mass 79.904. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Jump to main contentThe atomic number of each element increases by one, reading from left to .

The atomic number of each element increases by one, reading from left to .Fifty years ago bromine was produced on a massive scale and turned into lots of .

Bromine is the third halogen, being a nonmetal in group 17 of the periodic table. Its properties are thus similar to those of fluorine, chlorine, and iodine, and tend to be intermediate between those of the two neighbouring halogens, chlorine, and iodine. Bromine has the electron configuration [Ar]4s 3d 4p , with the seven electrons in the fourth and outermost shell acting as its valence electrons. Like all halogens, it is thus one electron short of a full octet, and is hence a strong oxidising age.
Therefore, the number of electrons in neutral atom of Bromine is 35. Each electron is influenced by the electric fields .
Learn how to write the complete electron configuration of bromine (Br) through orbitals and orbitals. The number of electrons in bromine is 35 and they are .Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine .Bromine is a halogen and a nonmetal with 35 electrons. Learn about its discovery, properties, uses, and ozone depletion effects.
Therefore, the number of electrons in neutral atom of Bromine is 35. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom.Bromine is a nonmetal with atomic number 35 and symbol Br. It has 35 electrons in its neutral state and can form various oxidation states. Learn more about its properties, .Number of Protons/Electrons: 35. Number of Neutrons: 45. Classification: Halogen. Crystal Structure: Orthorhombic. Density @ 293 K: 3.119 g/cm 3. Color: Red. Atomic Structure. Isotopes. Facts. Date of .
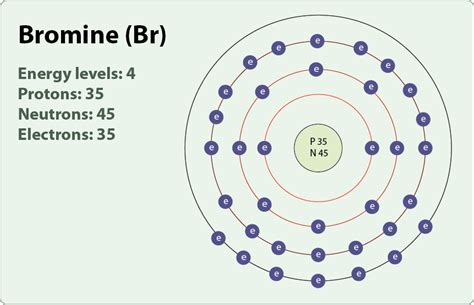
The electron arrangement in bromine atom is 2, 8, 18, 7. Hence, most commonly the bromine atom loses one electron during a chemical reaction. The most . Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br . Bromine is the third-lightest halogen, and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas.Bromine: Symbol: Br: Atomic Number: 35: . Number of Neutrons: 45: Number of Electrons: 35: Melting Point-7.2° C: Boiling Point: 58.78° C: Density: 3.119 grams per cubic centimeter: Normal Phase: Gas: Family: . Brominated vegetable oil; Citrus flavored soft drinks; Drug to aid in sleeplessness (no longer available in the United States) .number of electrons in bromine 4th shell can hold 32 electrons. Now the atomic number of bromine (Br) is 35. Hence the bromine element has electrons arrangement 2, 8, 18, 7. This electron arrangement indicates that the outermost orbit of Bromine element (Br) has 7 electrons. Hence, it lies in group 17.Iba pa 4th shell can hold 32 electrons. Now the atomic number of bromine (Br) is 35. Hence the bromine element has electrons arrangement 2, 8, 18, 7. This electron arrangement indicates that the outermost orbit of Bromine element (Br) has 7 electrons. Hence, it lies in group 17. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. The chemical symbol for Bromine is Br. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The nucleus is composed of protons and neutrons.
number of electrons in bromine Iba pa Protons. A proton is one of three main particles that make up the atom. Protons are found in the nucleus of the atom. This is a tiny, dense region at the center of the atom. Protons have a positive electrical charge of one ( + 1) and a mass of 1 atomic mass unit (amu), which is about 1.67 × 10 − 27 kilograms.Name: Bromine Symbol: Br Atomic Number: 35 Atomic Mass: 79.904 amu Melting Point:-7.2 °C (265.95 K, 19.04 °F) Boiling Point: 58.78 °C (331.93 K, 137.804 °F) Number of Protons/Electrons: 35 Number of Neutrons: 45 Classification: Halogen Crystal Structure: Orthorhombic Density @ 293 K: 3.119 g/cm 3 Color: Red Atomic StructureDetermine the total number of electrons in the valence shells of bromine atoms. The bromine molecule contains only one element. In the periodic table, bromine is a group VIIA element with seven electrons in its last shell. . Bromine has an electron configuration of 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 5 with the valence electrons in the 4s .
The mass of one atom is usually expressed in atomic mass units (amu), which is referred to as the atomic mass. An amu is defined as exactly 1/12 1 / 12 of the mass of a carbon-12 atom and is equal to 1.6605 × × 10 −24 g. Protons are relatively heavy particles with a charge of 1+ and a mass of 1.0073 amu. The number of valence electrons in one atom of each element is easily determined based on its position in the periodic table. For the main group elements (groups designated with a Roman numeral followed by the letter A), the number of valence electrons is equal to the Roman numeral. It would also be equal to the last digit of the . Since the atomic number of bromine is 35, the total electrons of bromine are 35. Second, make a table of subshell and its maximum electrons. Calculate the maximum number of electrons each subshell can hold using the formula: 4ℓ + 2. Where, ℓ = azimuthal quantum number of the subshell. For s subshell, ℓ = 0.The element Bromine was discovered by J. Balard and C. Löwig in year 1825 in France. Bromine was first isolated by J. Balard and C. Löwig in 1825. How many valence electrons does a Bromine atom have? .
Bromine (Br) Bromine is the 35th element in the periodic table and has a symbol of Br and atomic number of 35. It has an atomic weight of 79.904 and a mass number of 79. Bromine has thirty-five protons and forty-four neutrons in its nucleus, and thirty-five electrons in four shells. It is located in group seventeen, period four and block p of .
In this video we’ll use the Periodic table and a few simple rules to find the protons, electrons, and neutrons for the element Bromine (Br). From the Periodi. Bromine is the 35th element in the periodic table and the 3rd element in group 17. Bromine is a halogen element and its symbol is ‘Br’. The valence electrons are the total number of electrons in the last orbit (shell).. The total number of electrons in the last shell after the electron configuration of bromine is called the valence electrons of .
Make sure that you round the atomic mass to the nearest whole number. For example, the atomic mass of boron is 10.811, but you can just round the atomic mass up to 11. 6. Subtract the atomic number from the atomic mass. To find the number of neutrons, you will need to subtract the atomic number from the atomic mass.
The highest principal quantum number is 2. There are 2 electrons in the 2s subshell and 2 electrons in the 2 p subshell, giving carbon a total of four valence electrons. Bromine’s ground state electron configuration is 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 4p 5. The valence electrons are be the 4s and 4p electrons. Bromine has seven .Number of Electrons (with no charge): 35; Number of Neutrons (most common/stable nuclide): 45; Number of Protons: 35; Oxidation States: ±1,5; Valence Electrons: 4s 2 p 5 . Bromine - Br (EnvironmentalChemistry.com)- Comprehensive information for the element Bromine - Br is provided by this page including scores of properties, element .
Number of Neutrons = Mass Number - Number of Protons = 1 - 1 = 0. For zinc, the atomic weight is 65.39, so the mass number is closest to 65. Number of Neutrons = 65 - 30 = 35. Cite this Article. Follow these simple steps to find the number of protons, neutrons, and electrons for an atom of any element.
number of electrons in bromine|Iba pa
PH0 · number of neutrons in br
PH1 · number of electrons in boron
PH2 · number of electrons in an atom
PH3 · etron vape pen
PH4 · element with 4 electrons
PH5 · calculating protons neutrons and electrons
PH6 · Iba pa